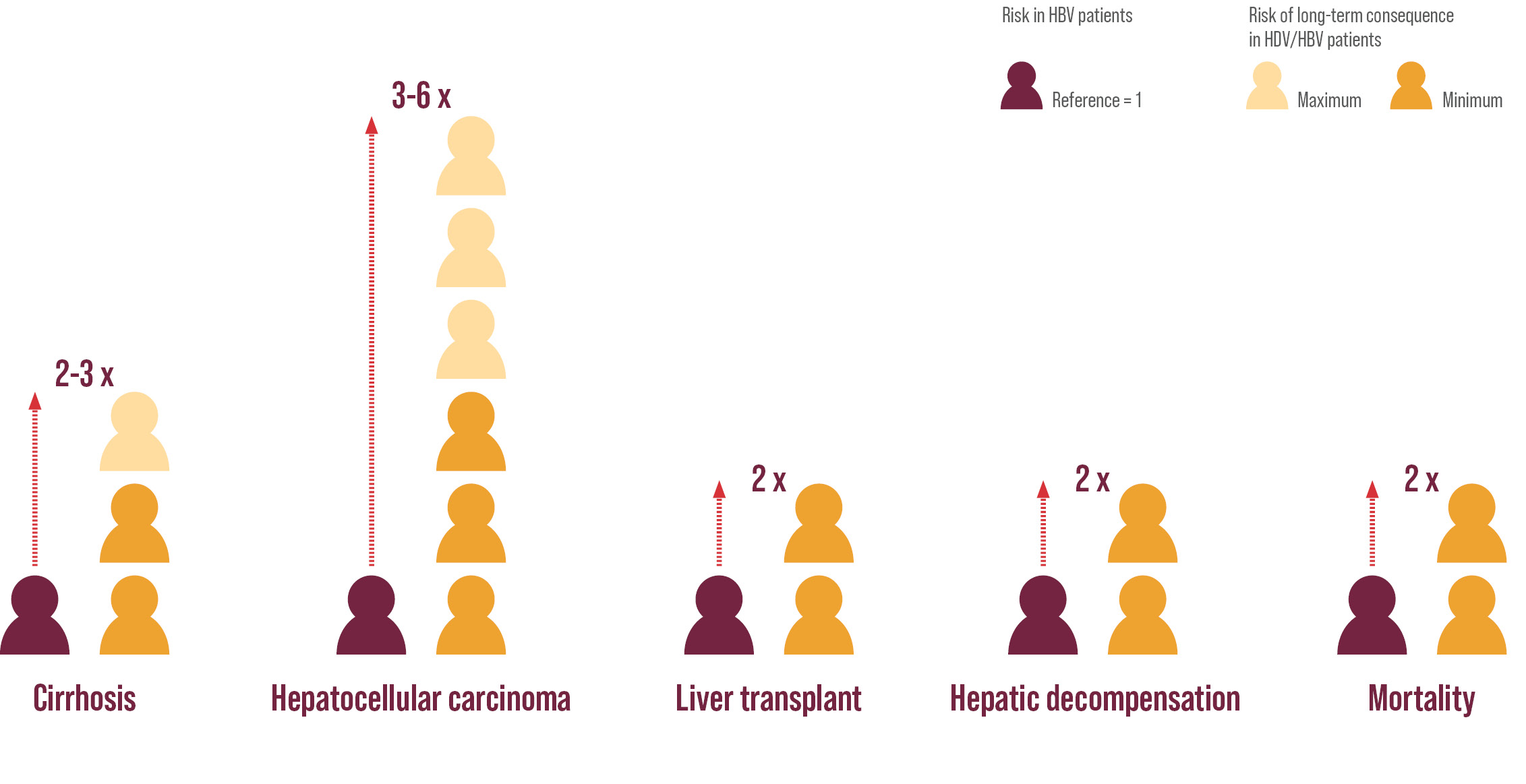
Compared with HBV-monoinfected patients, patients also infected with hep delta have 2-3x more likely to develop cirrhosis; 3-6x higher risk for hepatocellular carcinoma; 2x more likely to develop hepatic decompensation, liver transplantation or death3.
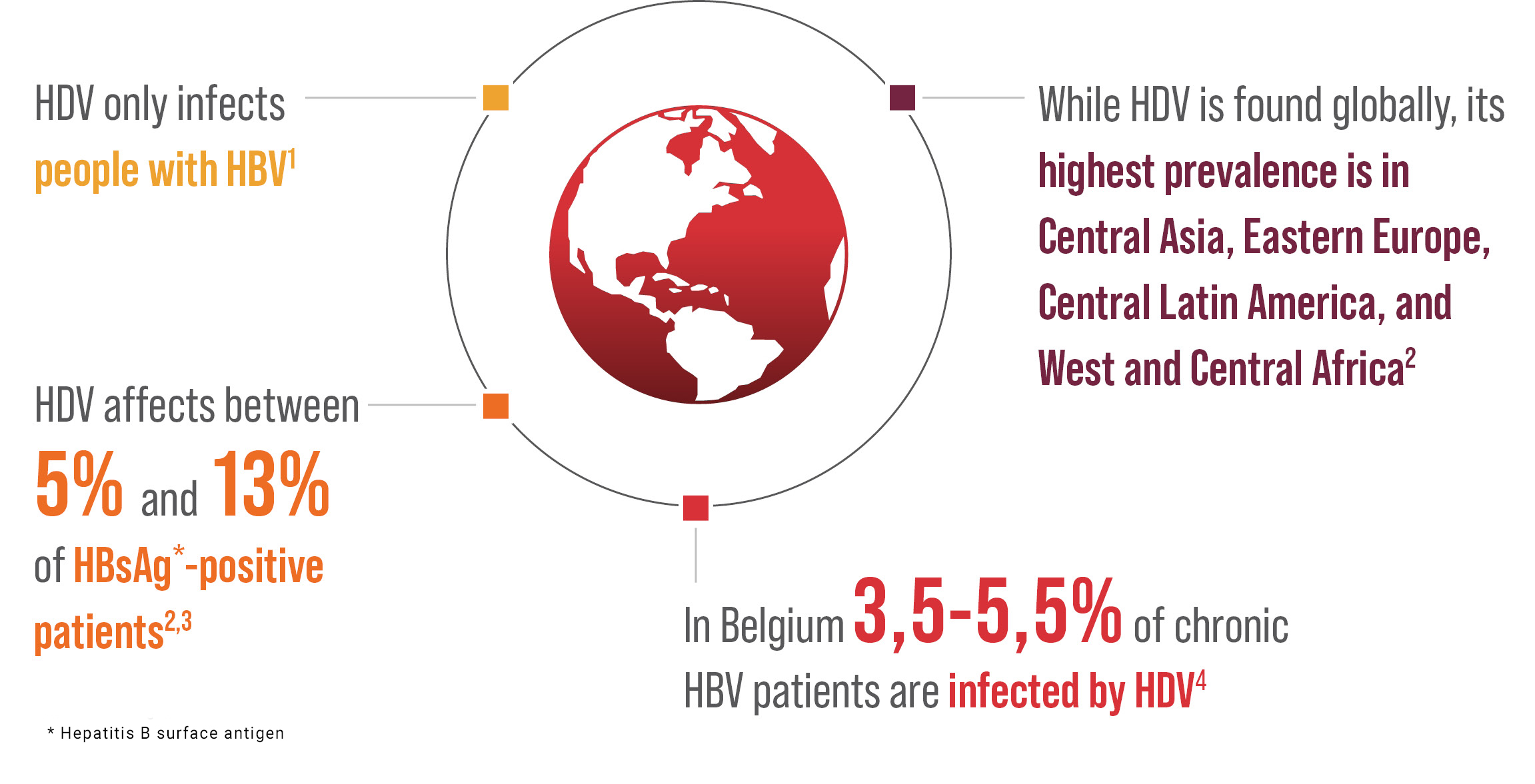
Recent data suggest that hepatitis delta virus affects 12-60 millions of people around the globe.
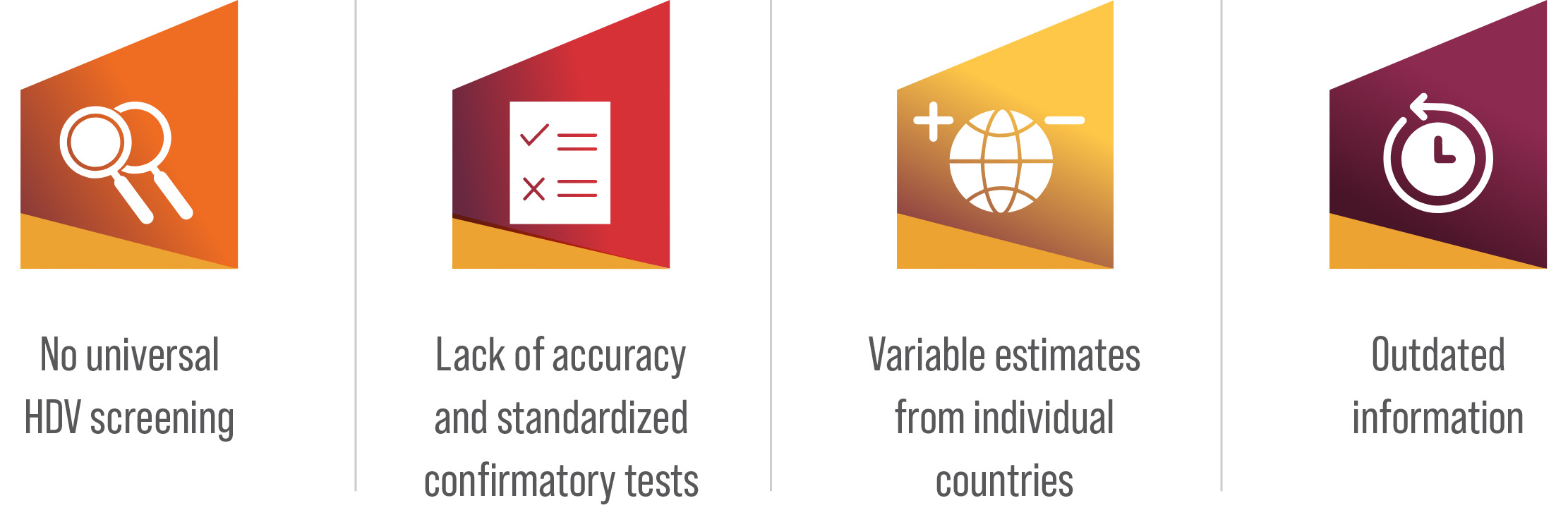
Several factors contribute to the lack of accurate estimates.
To help you better understand the mechanism of the disease, watch the video below:
HDV requires HBV surface antigen (HBsAg) as a surface protein to disseminate infection inside liver cells6
Once inside, the hepatitis delta virus genome (1.7 kb) is too small to code for the proteins required for its own replication and relies on the host machinery of liver cells to divide6
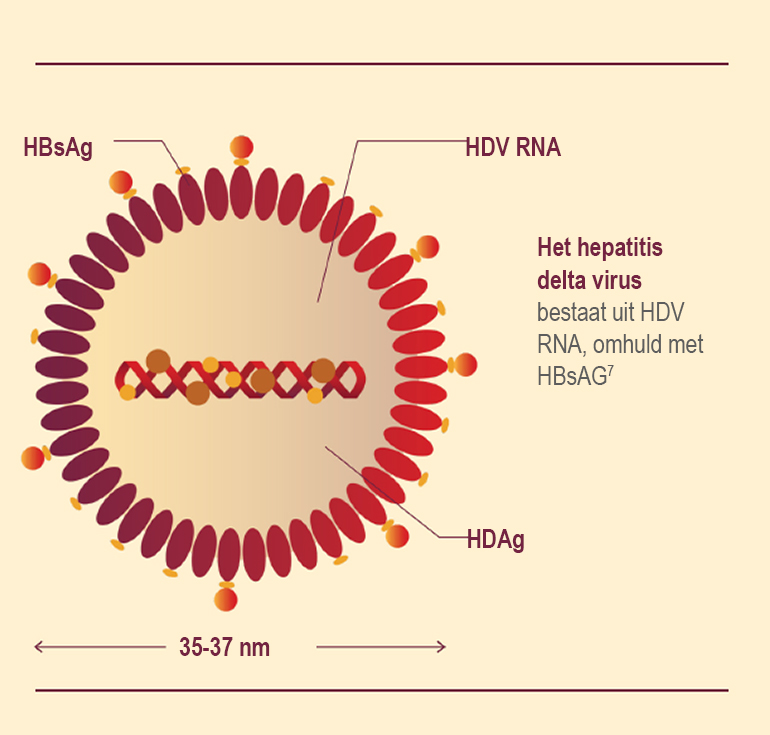
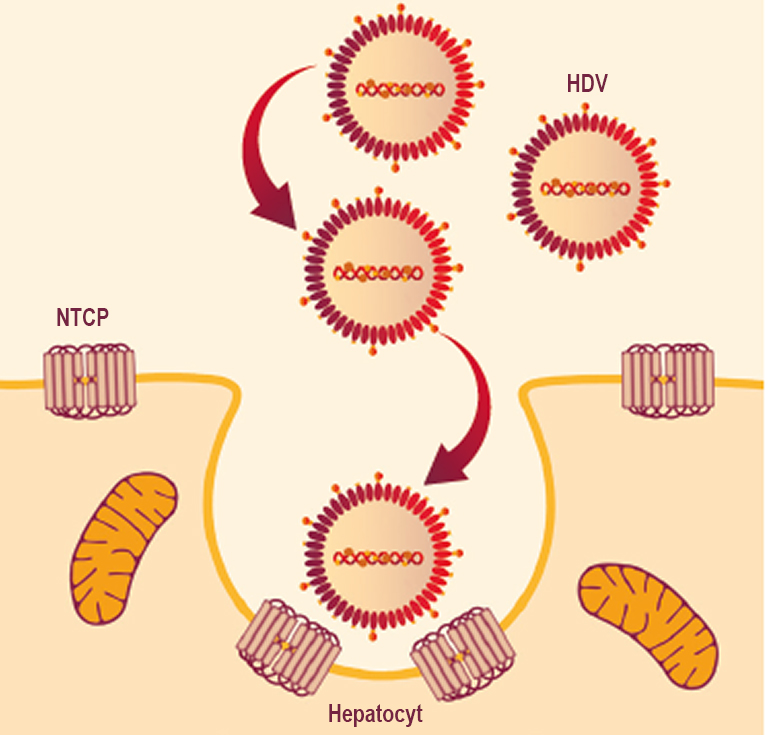
HDV gains entry into uninfected hepatocytes using the HBsAg envelope
HDV tends to suppress HBV replication.
However, HBV viral load does not affect HDV viral load.
Treatment with NAs is ineffective in HDV.
Contact with infected blood
Screening HBV patients helps identify those who can be offered appropriate management11
EASL guidelines recommend that ALL HBV-infected patients should be screened for HDV 12
Recommendations AFEF 202013
Screening and diagnosis for HDV involves two steps:
A blood test, called an HDV antibody test, is used to find out if someone has ever been infected with the hepatitis D virus. The HDV antibody test, sometimes called the anti-HDV test, looks for antibodies to the hepatitis D virus in blood. Antibodies are chemicals released into the bloodstream when someone gets infected. Test results can take anywhere from a few days to a few weeks to come back.
If the antibody test is reactive or positive, you need an additional test to see if you currently have hepatitis D. This test is called a nucleic acid test (NAT) for HDV RNA. Another name used for this test is a PCR test.
If the HDV RNA test is positive - you now have the virus in your blood.
Reduction in HDV RNA levels, which typically leads to a normalization of ALT levels16
Improvement of quality of live
Control disease progression
Achieving treatment goals aims to prevent long-term complications of HDV, including7:
NL-UNB-0857