
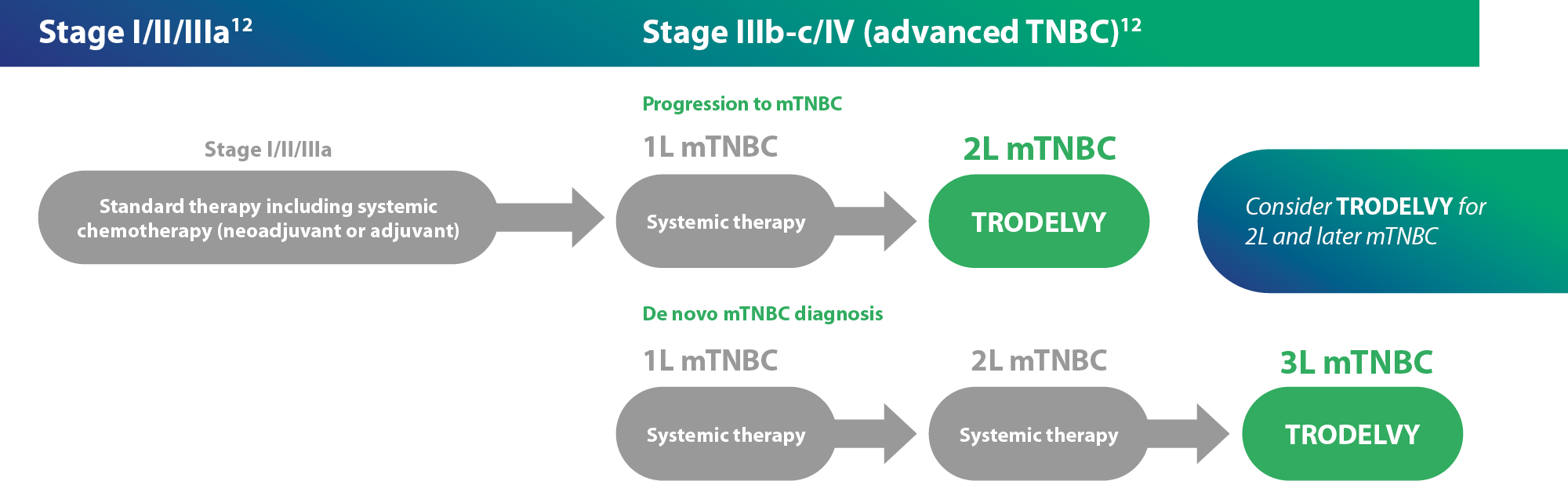
TRODELVY® as monotherapy is reimbursed for the treatment of adult patients with unresectable or metastatic triple-negative breast cancer (mTNBC) who have received two or more prior systemic therapies, including at least one of them for advanced disease10,11,12
▼ This medical product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
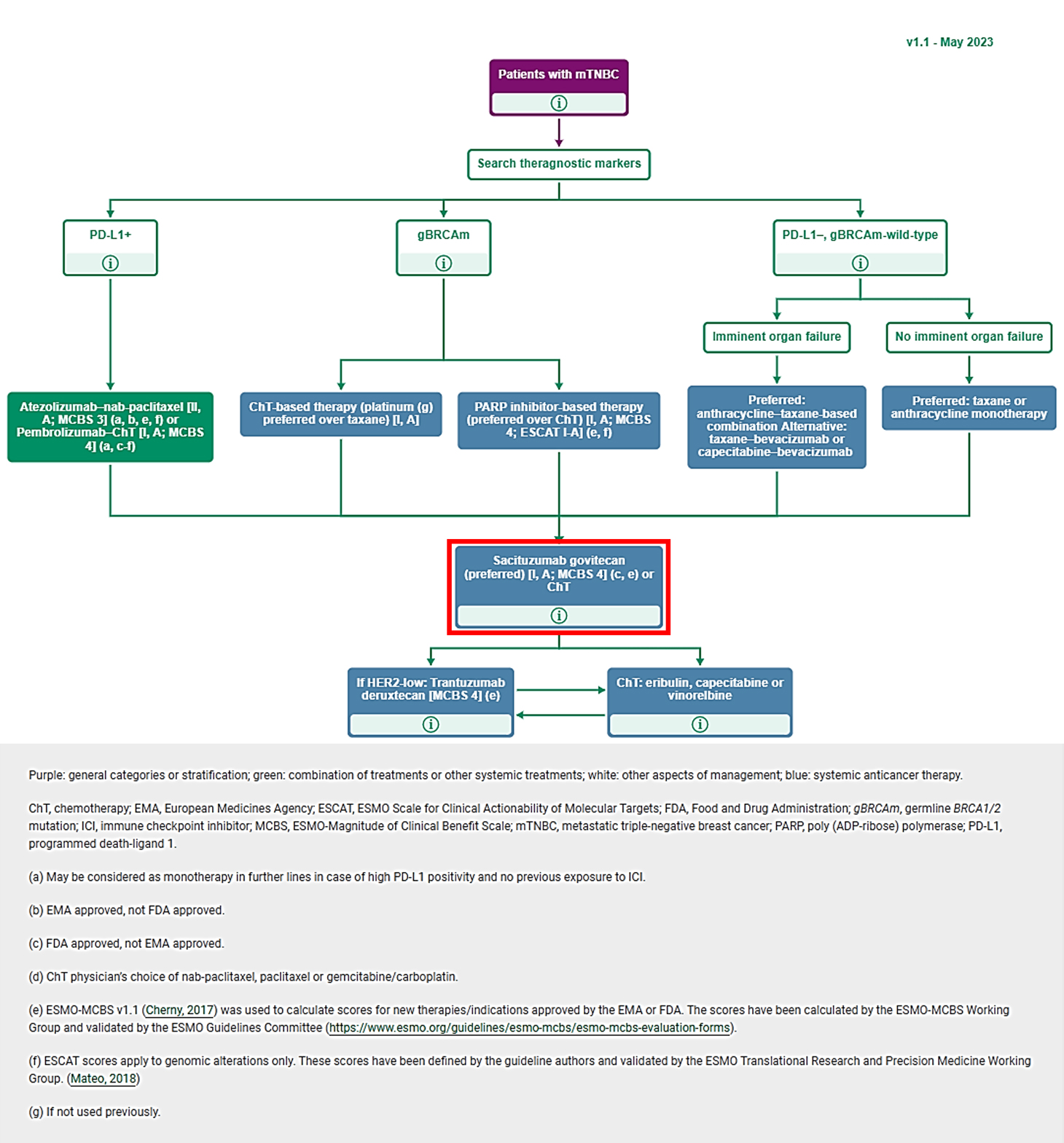
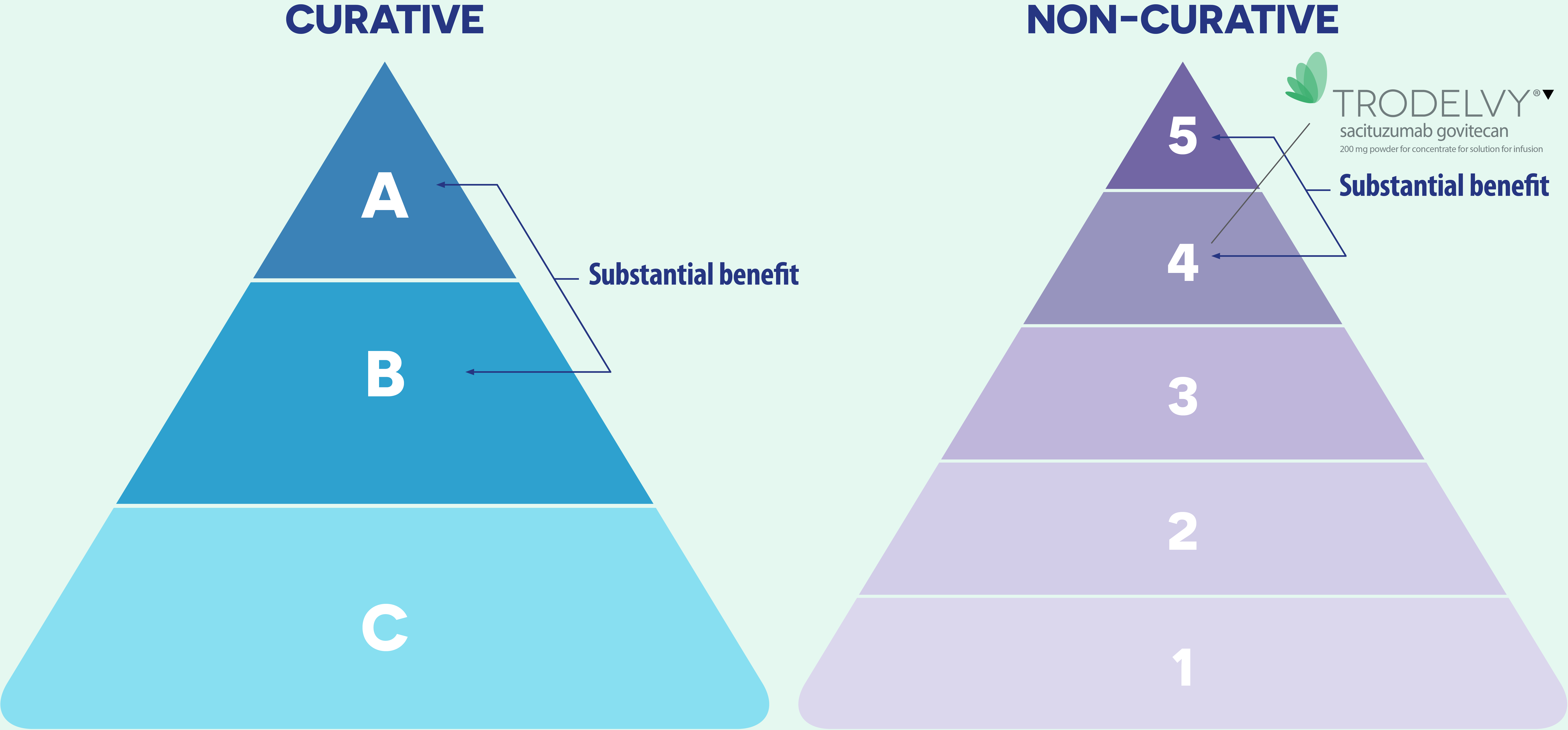
ESMO-Magnitude of clinical benefit scale (ESMO-MCBS) was developed in 2015 as a tool to assess the magnitude of clinical benefit from new anti-cancer therapies and has been widely applied in clinical and in health technology assessment settings.
Based on clinical trials results, the scale considers – in the curative and non-curative settings - overall survival, progression-free survival, disease free survival, hazard ratio, response rate, quality of life, prognosis of the condition and toxicity.
Treatments that have an ESMO-MCBS score of 4 and 5 for non-curative therapies are highlighted for accelerated assessment of value and cost-effectiveness.
Based on the ESMO-MCBS evaluation, TRODELVY® obtained a score of 4 out of 5 demonstrating a significant clinical benefit for the patients with metastatic triple negative breast cancer.
TRODELVY® dellvers a potent cytotoxic payload combined with a TROP-2 directed antibody to mTNBC cells tumours14,15,16,17
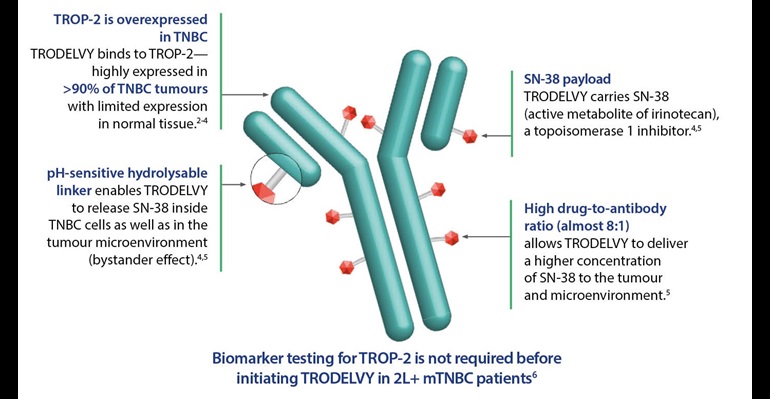
2L+, second line and beyond; ADC, antibody-drug conjugate; mTNBC, metastatic triple-negative breast cancer; SN-38, active metabolite of irinotecan; TNBC, triple-negative breast cancer; TROP-2, trophoblast cell surface antigen 2.
2. Bardia A, et al. N Engl J Med. 2021;384(16):1529-1541. 3. Shvartsur A, Bonavida B. Genes Cancer. 2015;6(3-4):84-105. 4. Goldenberg DM, et al. Oncotarget. 2015;6(26):22496-22512. 5. Rugo HS, et al. Future Oncol. 2020;16(12):705-715. 6. TRODELVY® (sacituzumab govitecan) Summary of Product Characteristics. Gilead Sciences Belgium.
TRODELVY® is a paradigm shifting TROP-2-Directed ADC that concentrates cytotoxic playload intracellularly while concurrently facilitating its extracellular release into the tumor microenvironment
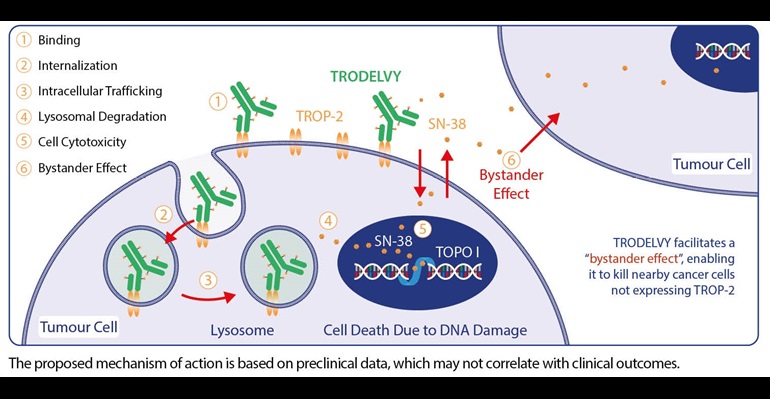
ADC, antibody-drug conjugate; SN-38, active metabolite of irinotecan; TNBC, triple-negative breast cancer; TOPO I, topoisomerase 1; TROP-2, trophoblast cell surface antigen 2.
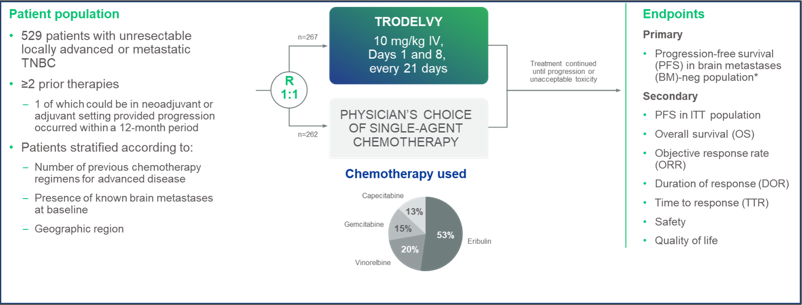
*PFS measured by an independent centralized and blinded group of radiology experts who assessed tumor response using RECIST 1.1 criteria in patients without brain metastasis. (n=468)
Efficacy
Median PFS with TRODELVY® was nearly 3X LONGER than chemotherapy22
Progression-free Survival*22
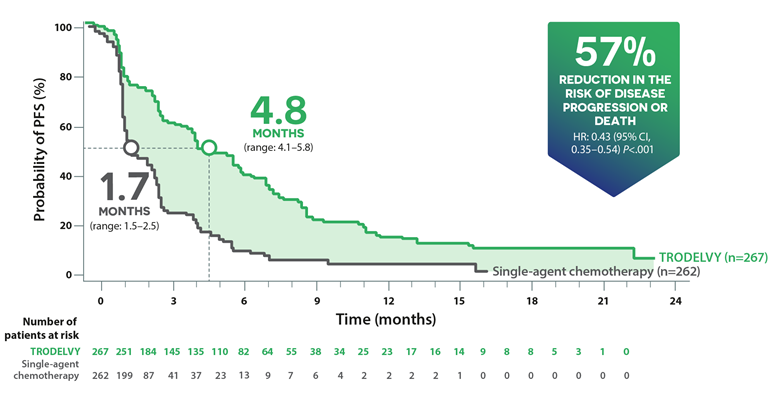
The median PFS in the total ITT population (all patients with or without brain metastases) was 4.8 months on TRODELVY® versus 1.7 months on chemotherapy (HR: 0.43; p<0.001).22
The improvement in PFS in patients without known brain metastases was consistent with that observed for the overall ITT population (5.6 months on treatment with TRODELVY® versus 1.7 months on chemotherapy (HR: 0.41; p<0.001).
The primary analysis population consisted of patients without known brain metastases at baseline (n=468).
The ITT population consisted of all patients with or without brain metastases at baseline (n=529).
Median OS of 1 YEAR with TRODELVY®22
Overall survival*22
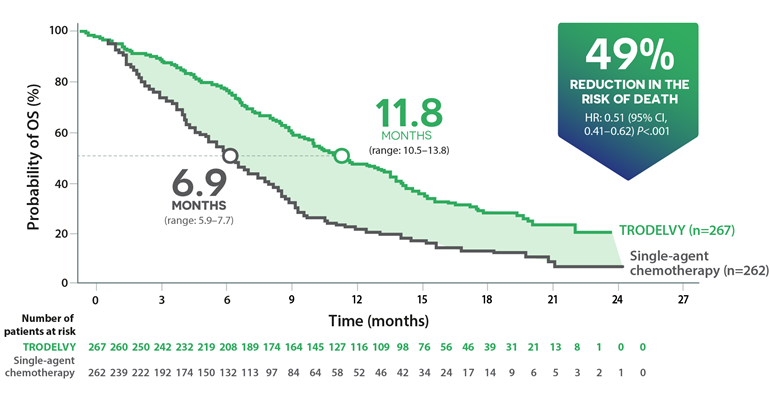
The median OS in the total ITT population (all patients with or without brain metastases) was 11.8 months on treatment with TRODELVY® versus 6.9 months on chemotherapy (HR: 0.51; p<0.001).22
The improvement in OS in patients without known brain metastases was consistent with that observed for the overall ITT population (12.1 months on treatment with TRODELVY® versus 6.7 months on chemotherapy (HR: 0.48; p<0.001).
The primary analysis population consisted of patients without known brain metastases at baseline (n=468).
The ITT population consisted of all patients with or without brain metastases at baseline (n=529).
TRODELVY® delivered NEARLY 8X GREATER response rate than chemotherapy22
Overall response rate+22
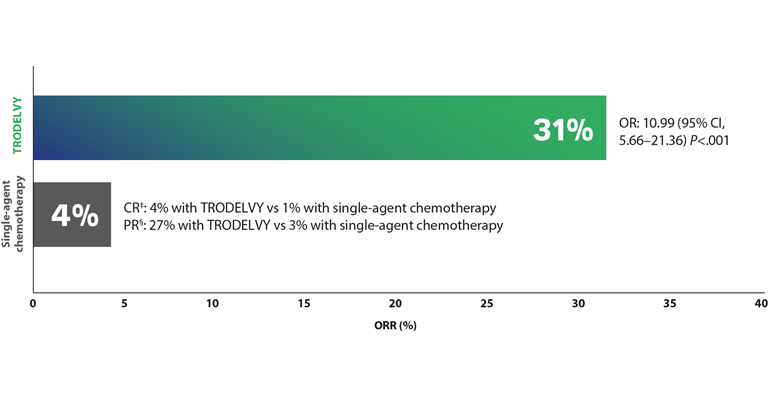
The ORR in the total ITT population (all patients with or without brain metastases) was 31% on treatment with TRODELVY® versus 4% on chemotherapy (OR:10.99 (95% CI, 5.66-21.36) p<.001).22
†The ORR results in the primary analysis population were consistent with the ITT population (OR: 35% vs. 5%; OR: 10.86; 95% CI, 5.59-21.0). The primary analysis population consisted of patients without brain metastases at baseline (n=468). The ITT population consisted of all patients with or without brain metastases at baseline (N=529).22
The median duration of response was 6.3 months with TRODELVY® (range: 5.5-9.0) versus 3.6 months with single agent chemotherapy (range: 2.8-NE).22
*Based on objective response rates for TRODELVY® and single-agent chemotherapy.
‡Complete response (CR): disappearance of all target lesions. All pathological lymph nodes (both target and non-target lesions) should be reduced to <10 mm on the short axis.29
§Partial response (PR): at least 30% reduction in the total diameter of the target lesions, using the total sum of diameters at baseline as a reference.29
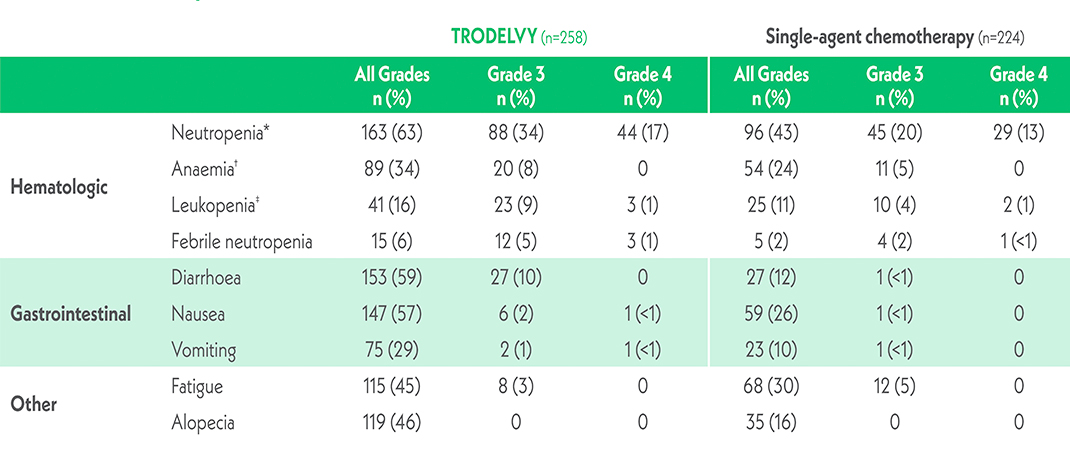
"Adverse events leading to treatment discontinuation were infrequent, occurring in 12 patients (5%) in each group."
Bardia A, Hurcitz SA, Tolaney SM, et al; for the ASCENT Clinical Trial Investigators
For the full list of side effects please refer to SmPC
*The neutropenia category included neutropenia and decreased neutrophil count.
†The anaemia category included anaemia, decreased hemoglobin level, and decreased red-cell count.
‡The leukopenia category included leukopenia and decreased white-cell count.
In the Phase 3 ASCENT Trial:
No patients in the TRODELVY group discontinued treatment due to treatment-related neutropenia or diarrhoea22
*Based on pooled data from two clinical trials (ASCENT and IMMU-132-01) involving 366 patients who received TRODELVY®.1
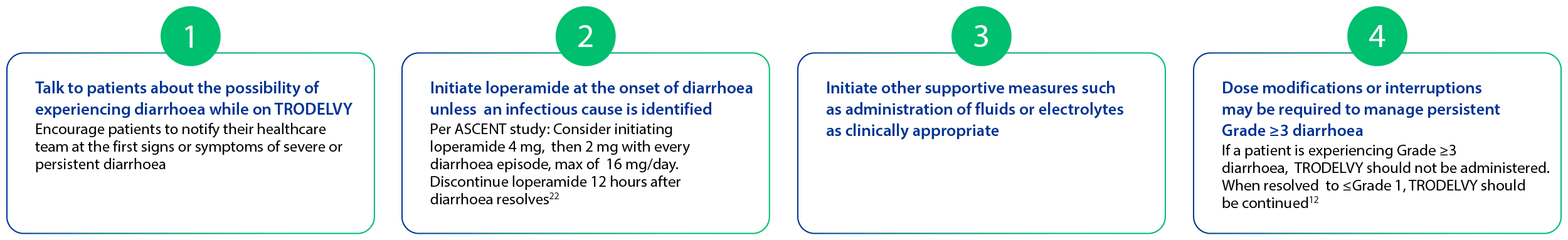
Early detection and proactive management can help control side effects
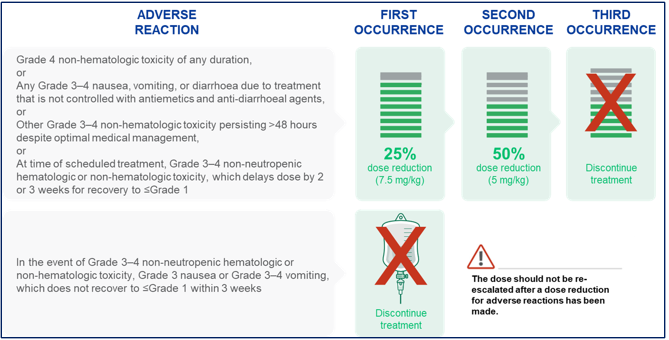
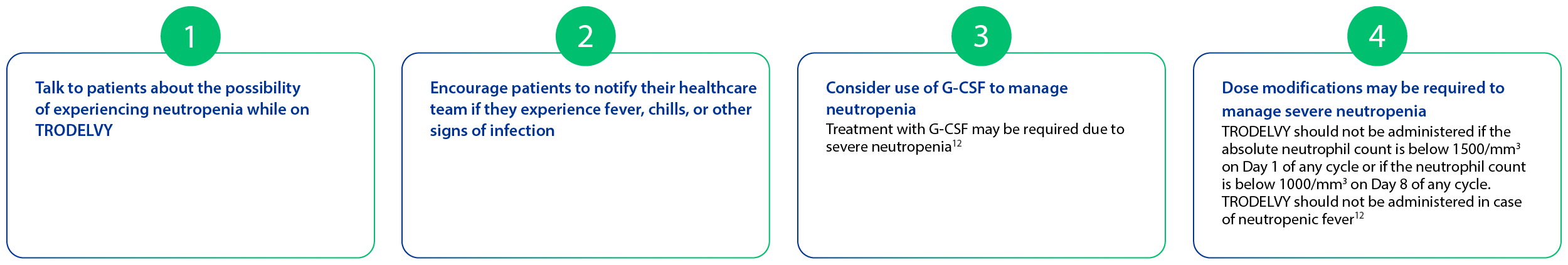
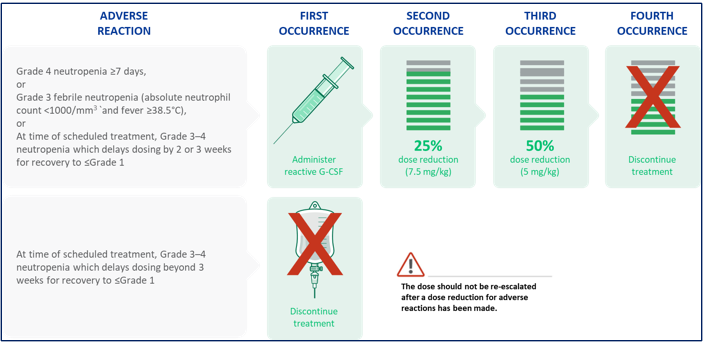
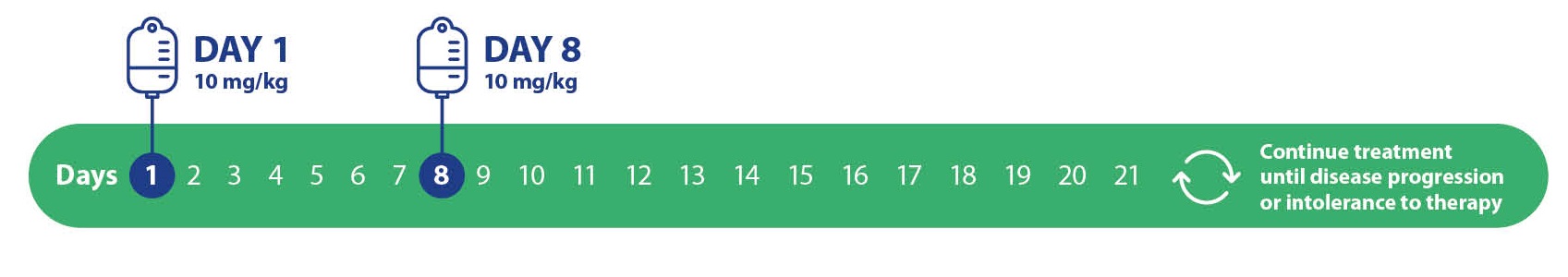
The recommended dose of TRODELVY® is 10 mg/kg intravenously on Days 1 and 8 of 21-day treatment cycles. Dose is calculated based on patient body weight at the beginning of each cycle9,10,11,12

2L+, second line and beyond; ADC, antibody-drug conjugate; AR, adverse reaction; BICR, blinded independent central review; CI, confidence interval; HR, hazard ratio; HRQoL, health-related quality of life; ITT, intent-to-treat; IV, intravenous; mTNBC, metastatic triple-negative breast cancer; NE, not estimable; OR, odds ratio; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumours; TNBC, triple-negative breast cancer; TROP-2, trophoblast cell surface antigen 2; QoL, quality of life; UGT1A1, uridine diphosphate-glucuronosyl transferase 1A1.
BE-TRO-0080